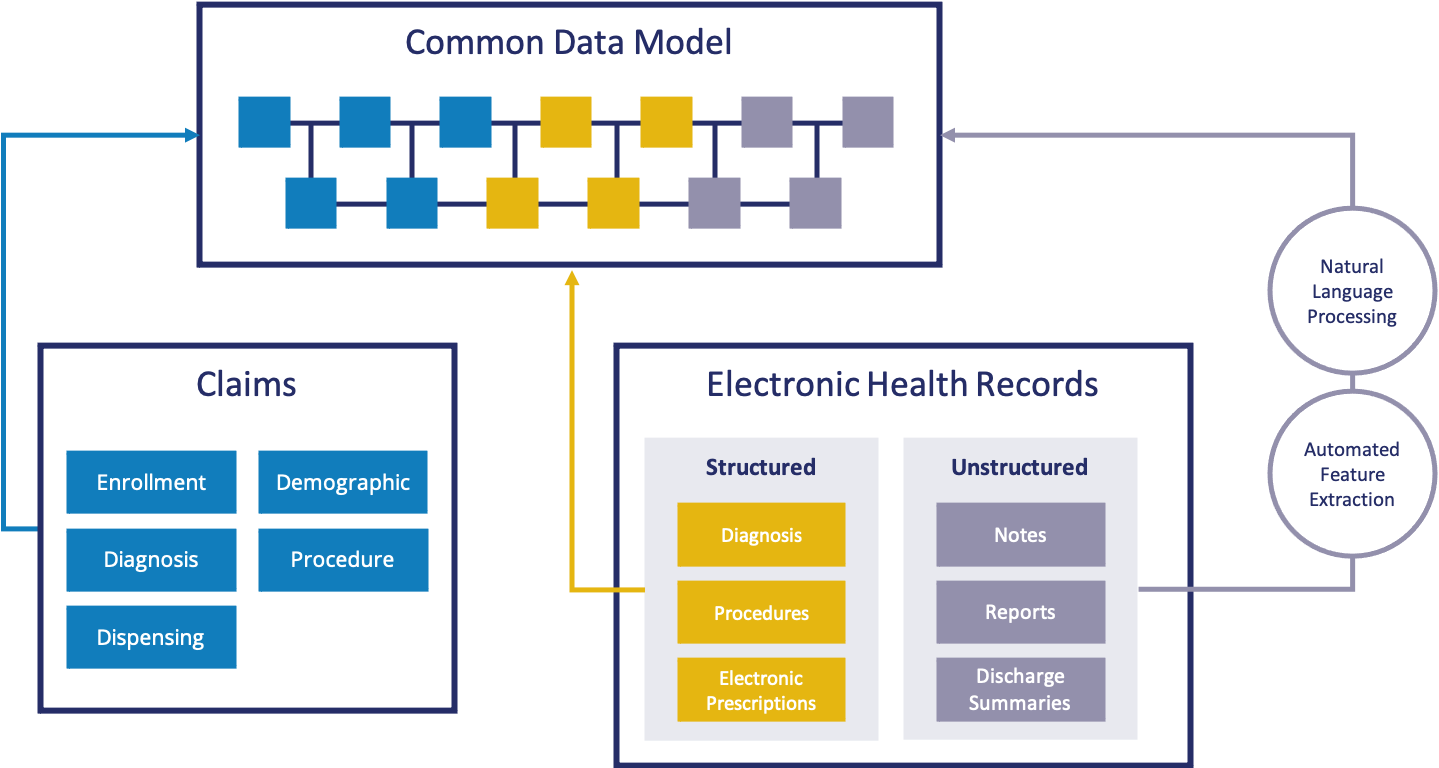
The Sentinel Innovation Center is a test bed to identify, develop, and evaluate innovative methods to study drug safety and effectiveness using real-world data. The IC was created in response to FDA’s Medical Data Enterprise Initiative to build a new system containing electronic health records from 10 million lives and the Sentinel Five-Year Strategy. Achieving this vision requires:
- Developing new analysis tools for unstructured EHR data
- Establishing novel data sources by finding new ways to extract, standardize and quality check clinical data and free-text in EHRs
- Creating state-of-the-art approaches to identify clinical phenotypes, extract key data elements and adjust for confounding in drug safety and effectiveness studies
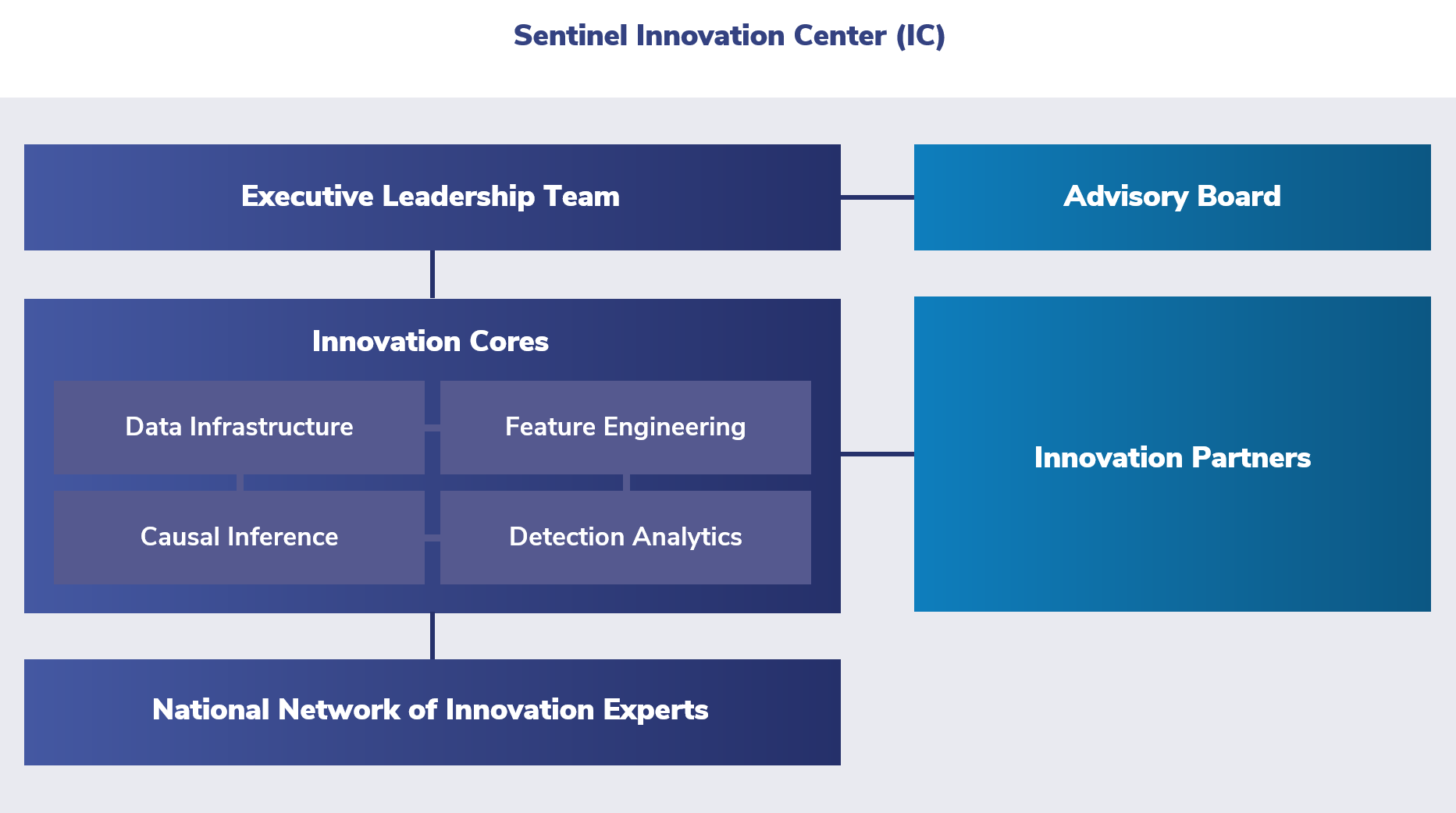
Leadership








Objectives
The Innovation Center was created to attract new data partnerships, engage external investigators to develop new methods, and grow the types of questions that can be addressed in the Sentinel System.
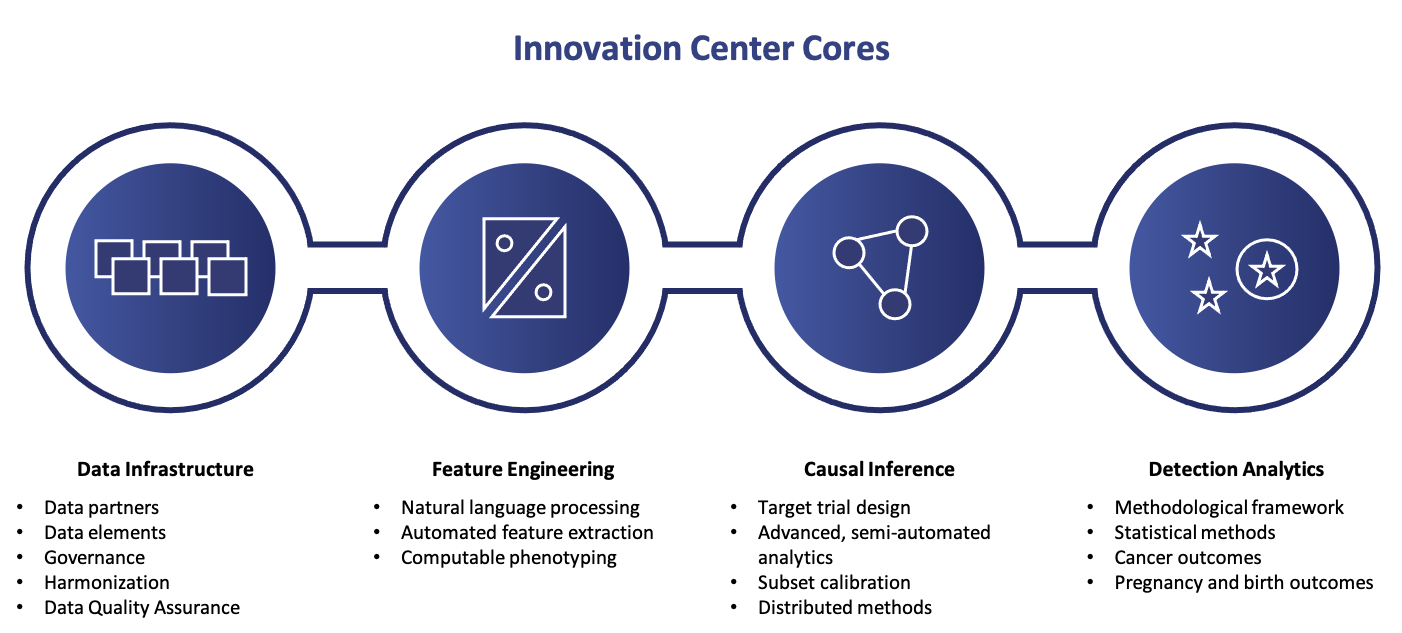
The IC has developed a scientific blueprint for the next five years, named the IC Master Plan. The Master Plan has four key strategic priority areas:
- Data infrastructure
- Feature engineering
- Causal inference
- Detection analytics
The first two priority areas help to establish a query-ready distributed data network containing electronic health records. The second two priorities help develop and evaluate new methods that can be transformed into reusable analytic tools.